 RI (high-affinity Fc receptor for IgE) and CD23, as well as galectin-3 and several co-receptors for CD23, notably CD21 and various integrins. Here, we review recent progress in uncovering the structures of these proteins and their complexes, and in our understanding of how IgE exerts its effects and how its expression is regulated. The information that has emerged suggests new therapeutic directions for combating allergic disease.
RI (high-affinity Fc receptor for IgE) and CD23, as well as galectin-3 and several co-receptors for CD23, notably CD21 and various integrins. Here, we review recent progress in uncovering the structures of these proteins and their complexes, and in our understanding of how IgE exerts its effects and how its expression is regulated. The information that has emerged suggests new therapeutic directions for combating allergic disease.Summary
- Remarkable progress has been made in recent years on the structural determination of proteins in the IgE network and on the functions and regulation of IgE. This is beginning to feed into IgE-targeted therapies for allergy.
- The shape of the IgE molecule differs dramatically from that of IgG. X-ray and nuclear magnetic resonance studies have also revealed conformational changes that occur on IgE binding to its high-affinity receptor Fc
 RI (high-affinity Fc receptor for IgE) on mast cells and antigen-presenting cells, events that lead, respectively, to sensitization (and the immediate hypersensitivity reaction) and the facilitation of allergen presentation.
RI (high-affinity Fc receptor for IgE) on mast cells and antigen-presenting cells, events that lead, respectively, to sensitization (and the immediate hypersensitivity reaction) and the facilitation of allergen presentation. - The structural data also provide clues to the unique nature of the high-affinity IgE–Fc
 RI interaction, and indicate possibilities for blocking the interaction; validation of IgE as a target is demonstrated by the success of the IgE-specific monoclonal antibody omalizumab in the treatment of asthma.
RI interaction, and indicate possibilities for blocking the interaction; validation of IgE as a target is demonstrated by the success of the IgE-specific monoclonal antibody omalizumab in the treatment of asthma. - The presence of an unusually long extracellular membrane-proximal domain in membrane IgE may also determine its ability to act as an antigen receptor on B cells and to respond to particular antigens (allergens).
- The trimeric structure of the C-type lectin, low-affinity IgE receptor CD23, and its susceptibility to cleavage by ADAM10 (a disintegrin and metalloproteinase 10) at the cell surface, provide important clues to the mechanism of IgE homeostasis.
- CD23 has multiple ligands, including IgE, CD21 and various integrins, enabling it to carry out several other functions, including IgE-dependent antigen presentation and cellular cytotoxicity.
- IgE is transported to mucosal tissues by CD23 and is also synthesized by the resident B cells. Its concentration is maintained in the tissue by the number of mast cells that express Fc
 RI at high levels and the slow rate of dissociation of IgE from Fc
RI at high levels and the slow rate of dissociation of IgE from Fc RI.
RI. - Class switching to IgE and affinity maturation of the antibodies occur in mucosal tissues, and this may limit the ability of IgE antibodies to mediate systemic anaphylaxis.
- Various mechanisms contrive to suppress the production of IgE synthesis to tolerable levels, as well as limiting its anatomical distribution. Some mechanisms operate at the level of class-switch recombination, others at the level of survival of the IgE-switched cells.
- IgE transport in both directions through the gastrointestinal epithelium may be involved in early sensitization to allergens. Studies of the mechanism of early sensitization may suggest means of preventing the development of allergic disease.
- Small molecule inhibitors of the IgE–Fc
 RI interaction may supersede IgE-specific antibodies, but the combination of this approach with immunotherapy may be required for more effective therapeutic intervention in allergy and asthma.
RI interaction may supersede IgE-specific antibodies, but the combination of this approach with immunotherapy may be required for more effective therapeutic intervention in allergy and asthma.
 RI (high-affinity Fc receptor for IgE) and CD23, as well as the IgE- and Fc
RI (high-affinity Fc receptor for IgE) and CD23, as well as the IgE- and Fc RI-binding protein galectin-3. In addition, the function of CD23 is extended by several co-receptors. These comprise the complement receptors CD21 (also known as CR2),
RI-binding protein galectin-3. In addition, the function of CD23 is extended by several co-receptors. These comprise the complement receptors CD21 (also known as CR2),  M
M 2-integrin (otherwise known as CD18/CD11b or CR3) and
2-integrin (otherwise known as CD18/CD11b or CR3) and  X
X 2-integrin (also known as CD18/CD11c or CR4); the vitronectin receptor (also known as
2-integrin (also known as CD18/CD11c or CR4); the vitronectin receptor (also known as  V
V 3-integrin); and
3-integrin); and  V
V 5-integrin. Here, we review the remarkable progress of the past several years on the structural determination of these proteins and of the complexes formed between them, and on the functions and regulation of IgE in the context of the extended protein network. The success of the IgE-specific antibody, omalizumab (Xolair; Novartis Pharmaceuticals Ltd), in the treatment of asthma and other allergic diseases demonstrates the virtues of targeting IgE for therapy5. We expect that a deeper understanding of the IgE network will lead to a new generation of IgE-targeted therapies.
5-integrin. Here, we review the remarkable progress of the past several years on the structural determination of these proteins and of the complexes formed between them, and on the functions and regulation of IgE in the context of the extended protein network. The success of the IgE-specific antibody, omalizumab (Xolair; Novartis Pharmaceuticals Ltd), in the treatment of asthma and other allergic diseases demonstrates the virtues of targeting IgE for therapy5. We expect that a deeper understanding of the IgE network will lead to a new generation of IgE-targeted therapies.Structures and interactions in the IgE network
 -chain contains one more domain than the heavy
-chain contains one more domain than the heavy  -chain of IgG. The pair of C
-chain of IgG. The pair of C 3 and C
3 and C 4 domains are homologous in sequence, and similar in quaternary structure, to the pair of C
4 domains are homologous in sequence, and similar in quaternary structure, to the pair of C 2 and C
2 and C 3 domains of IgG, so that it is the pair of C
3 domains of IgG, so that it is the pair of C 2 domains, located in the position equivalent to that occupied by the flexible hinge region of IgG, that is the most obvious distinguishing feature of IgE. Initially it was thought that this 'extra' domain pair might simply act as a spacer between the antigen-binding Fab 'arms' and the C
2 domains, located in the position equivalent to that occupied by the flexible hinge region of IgG, that is the most obvious distinguishing feature of IgE. Initially it was thought that this 'extra' domain pair might simply act as a spacer between the antigen-binding Fab 'arms' and the C 3–C
3–C 4 part of the Fc region (Fc
4 part of the Fc region (Fc 3-4), but elegant studies using fluorescence resonance energy transfer (FRET) determined that the distance between the N- and C-termini of the molecule was less than half that expected for an extended Y-shaped structure (6.9 Å compared with 17.5 Å)6; furthermore, this was also true for the IgE molecule bound to Fc
3-4), but elegant studies using fluorescence resonance energy transfer (FRET) determined that the distance between the N- and C-termini of the molecule was less than half that expected for an extended Y-shaped structure (6.9 Å compared with 17.5 Å)6; furthermore, this was also true for the IgE molecule bound to Fc RI (Refs 6, 7).
RI (Refs 6, 7). 2 domains, was determined, the molecule was found to be even more acutely bent than had been anticipated, with the C
2 domains, was determined, the molecule was found to be even more acutely bent than had been anticipated, with the C 2 domains folded back and making extensive contact with the C
2 domains folded back and making extensive contact with the C 3 domains, and even touching the C
3 domains, and even touching the C 4 domains8 (Fig. 2a, b). The structure was entirely consistent with X-ray and neutron scattering data obtained in solution9, showing that this compact, bent conformation was indeed its native structure, and was markedly different from the extended and flexible structure of IgG7. Nevertheless, within this overall compact structure, IgE displays considerable conformational flexibility both in tertiary (intra-domain) and quaternary (inter-domain) structure. Comparison of crystal structures of Fc
4 domains8 (Fig. 2a, b). The structure was entirely consistent with X-ray and neutron scattering data obtained in solution9, showing that this compact, bent conformation was indeed its native structure, and was markedly different from the extended and flexible structure of IgG7. Nevertheless, within this overall compact structure, IgE displays considerable conformational flexibility both in tertiary (intra-domain) and quaternary (inter-domain) structure. Comparison of crystal structures of Fc 3-4 alone and in complex with Fc
3-4 alone and in complex with Fc RI (Ref. 10) (see below) show that the C
RI (Ref. 10) (see below) show that the C 3 domains can adopt 'closed' (Fig. 2c) or 'open' (Fig. 2d) conformations by rotating relative to C
3 domains can adopt 'closed' (Fig. 2c) or 'open' (Fig. 2d) conformations by rotating relative to C 4. The asymmetrically bent IgE-Fc (Fig. 2a, b), with its C
4. The asymmetrically bent IgE-Fc (Fig. 2a, b), with its C 2 domains packed against the two C
2 domains packed against the two C 3 domains, has one C
3 domains, has one C 3 domain in the 'open' conformation, while the other is 'closed', and this structural asymmetry explains a key feature of the interaction with Fc
3 domain in the 'open' conformation, while the other is 'closed', and this structural asymmetry explains a key feature of the interaction with Fc RI, as we discuss later.
RI, as we discuss later. |
| FIGURE 2 | The structures of IgE-Fc fragments.
a, b | Two (approximately orthogonal) views of the complete IgE-Fc (uncomplexed) including the C
 2 domains (Protein Data Bank (PDB) ID: 1O0V). The two 2 domains (Protein Data Bank (PDB) ID: 1O0V). The two  -chains are coloured purple and green, and the two points of connection to the Fab regions can be seen most clearly in a and b respectively, indicating the extent of the bend in the IgE molecule. c | The structure of the Fc -chains are coloured purple and green, and the two points of connection to the Fab regions can be seen most clearly in a and b respectively, indicating the extent of the bend in the IgE molecule. c | The structure of the Fc 3-4 fragment (uncomplexed) showing the two C 3-4 fragment (uncomplexed) showing the two C 3 domains in the 'closed' conformation (PDB ID: 1FP5). d | The structure of the Fc 3 domains in the 'closed' conformation (PDB ID: 1FP5). d | The structure of the Fc 3-4 fragment taken from the crystal structure of the complex of Fc 3-4 fragment taken from the crystal structure of the complex of Fc 3-4 and the Fc 3-4 and the Fc RI RI  -chain, showing the C -chain, showing the C 3 domains in the 'open' conformation (PDB ID: 1F6A). 3 domains in the 'open' conformation (PDB ID: 1F6A). |
 and Ig
and Ig constitutes the receptor for antigen on B cells committed to IgE synthesis. Each heavy chain of all membrane IgE molecules contains an additional extracellular membrane-proximal domain (EMPD) between the C
constitutes the receptor for antigen on B cells committed to IgE synthesis. Each heavy chain of all membrane IgE molecules contains an additional extracellular membrane-proximal domain (EMPD) between the C 4 domain and the transmembrane sequence, although there are at least two isoforms with EMPDs of different length,
4 domain and the transmembrane sequence, although there are at least two isoforms with EMPDs of different length,  short (14 amino acids) and
short (14 amino acids) and  long (66 amino acids)11. Nothing is known of the three-dimensional structure of the EMPDs, which contain inter-
long (66 amino acids)11. Nothing is known of the three-dimensional structure of the EMPDs, which contain inter- -chain disulphide bridges, nor the way in which IgE and its Fab regions are presented at the cell surface, but the two isoforms associate with different glycoforms of Ig
-chain disulphide bridges, nor the way in which IgE and its Fab regions are presented at the cell surface, but the two isoforms associate with different glycoforms of Ig and, together with other accessory proteins, form functionally distinct antigen receptors12. Whatever the position of the IgE molecule in this complex, the binding site for Fc
and, together with other accessory proteins, form functionally distinct antigen receptors12. Whatever the position of the IgE molecule in this complex, the binding site for Fc RI is accessible to receptors expressed on another cell surface; the implications of this interaction for allergic disease have yet to be explored13.
RI is accessible to receptors expressed on another cell surface; the implications of this interaction for allergic disease have yet to be explored13. RI and its interaction with IgE. Fc
RI and its interaction with IgE. Fc RI is expressed as an
RI is expressed as an 

 2 tetramer on mast cells and basophils, and as an
2 tetramer on mast cells and basophils, and as an 
 2 trimer on human but not mouse antigen-presenting cells (APCs), monocytes, eosinophils, platelets and smooth-muscle cells, but its distribution is even wider than this14, 15. The two extracellular domains of the
2 trimer on human but not mouse antigen-presenting cells (APCs), monocytes, eosinophils, platelets and smooth-muscle cells, but its distribution is even wider than this14, 15. The two extracellular domains of the  -chain (sFc
-chain (sFc RI
RI ) contain the IgE binding function, whereas the signalling motifs (immunoreceptor tyrosine-based activation motifs (ITAMs)) are located in the intracellular sequences of the
) contain the IgE binding function, whereas the signalling motifs (immunoreceptor tyrosine-based activation motifs (ITAMs)) are located in the intracellular sequences of the  - and
- and  -chains. Although the quaternary arrangement of the chains is not known, X-ray analyses of sFc
-chains. Although the quaternary arrangement of the chains is not known, X-ray analyses of sFc RI
RI in several different crystal forms have revealed that the two domains are folded back on each other exposing a hydrophobic 'ridge' (Fig. 3a) that was subsequently found to be the IgE binding site. Part of this ridge is formed by the CC' loop of the
in several different crystal forms have revealed that the two domains are folded back on each other exposing a hydrophobic 'ridge' (Fig. 3a) that was subsequently found to be the IgE binding site. Part of this ridge is formed by the CC' loop of the  2 domain, a segment that strikingly is the only region to exhibit conformational variation among the different unliganded sFc
2 domain, a segment that strikingly is the only region to exhibit conformational variation among the different unliganded sFc RI
RI structures16, 17.
structures16, 17. |
FIGURE 3 | The structure of the Fc RI RI  -chain and its complex with IgE. -chain and its complex with IgE.
a | The structure of the extracellular domains of the Fc
 RI RI  -chain (Protein Data Bank (PDB) ID: 1F6A), taken from the crystal structure of the Fc -chain (Protein Data Bank (PDB) ID: 1F6A), taken from the crystal structure of the Fc 3-4–Fc 3-4–Fc RI RI  -chain complex, with the superimposed structure of free Fc -chain complex, with the superimposed structure of free Fc RI RI  -chain (PDB ID: 1J87) showing only the region (shown in red) in which the structures differ. This is the CC' loop region, which displays conformational flexibility even within uncomplexed structures determined in different crystal forms. The structural change involves the edge -chain (PDB ID: 1J87) showing only the region (shown in red) in which the structures differ. This is the CC' loop region, which displays conformational flexibility even within uncomplexed structures determined in different crystal forms. The structural change involves the edge  -strand moving from one face of the immunoglobulin fold to the other. b | The structure of the high-affinity complex between Fc -strand moving from one face of the immunoglobulin fold to the other. b | The structure of the high-affinity complex between Fc 3-4 and the extracellular domains of the Fc 3-4 and the extracellular domains of the Fc RI RI  -chain, showing the extensive interaction surface and engagement of both C -chain, showing the extensive interaction surface and engagement of both C 3 domains in the 'open' conformation (PDB ID: 1F6A). The connection to the membrane is at the C-terminal end of the 3 domains in the 'open' conformation (PDB ID: 1F6A). The connection to the membrane is at the C-terminal end of the  2 domain. c | Schematic representation of the entire IgE molecule bound to the extracellular domains of the Fc 2 domain. c | Schematic representation of the entire IgE molecule bound to the extracellular domains of the Fc RI RI  -chain, according to the structural information from the Fc -chain, according to the structural information from the Fc 3-4 complex and the bent IgE-Fc structure. The 3-4 complex and the bent IgE-Fc structure. The  - and - and  -chains of Fc -chains of Fc RI, with their immunoreceptor tyrosine-based activation motifs (ITAMs), are also shown. RI, with their immunoreceptor tyrosine-based activation motifs (ITAMs), are also shown. |
 RI with IgE came from the Fc
RI with IgE came from the Fc 3-4–sFc
3-4–sFc RI
RI structure18, which showed how the two C
structure18, which showed how the two C 3 domains of IgE open up and how each contributes a sub-site of predominantly hydrophobic interaction surface (Fig. 3b). The location of this binding site clearly accounts for the observed 1:1 stoichiometry despite the presence of two identical
3 domains of IgE open up and how each contributes a sub-site of predominantly hydrophobic interaction surface (Fig. 3b). The location of this binding site clearly accounts for the observed 1:1 stoichiometry despite the presence of two identical  -chains, and the extensive hydrophobic buried surface explains the high affinity (Ka
-chains, and the extensive hydrophobic buried surface explains the high affinity (Ka  1010 M-1). In IgE (or IgE-Fc) with its C
1010 M-1). In IgE (or IgE-Fc) with its C 2 domains, one of the C
2 domains, one of the C 3 domains is already in an open configuration and therefore accessible for receptor binding, as described above, whereas the other is not. The conformational changes that are necessary to allow IgE engagement using both sub-sites must therefore also include the C
3 domains is already in an open configuration and therefore accessible for receptor binding, as described above, whereas the other is not. The conformational changes that are necessary to allow IgE engagement using both sub-sites must therefore also include the C 2 domains, and the effect of their presence on the kinetics of the interaction — a reduction in both the on-rate and off-rate of IgE binding — have been quantified19, 20. The kinetics are biphasic19, consistent with the involvement of two sub-sites. It may be necessary for these extensive structural changes in IgE, and the CC' loop of the receptor, to occur in a concerted way to allow IgE disengagement. This would account in part for the exceptionally slow dissociation rate compared to IgG from its structurally homologous receptors20.
2 domains, and the effect of their presence on the kinetics of the interaction — a reduction in both the on-rate and off-rate of IgE binding — have been quantified19, 20. The kinetics are biphasic19, consistent with the involvement of two sub-sites. It may be necessary for these extensive structural changes in IgE, and the CC' loop of the receptor, to occur in a concerted way to allow IgE disengagement. This would account in part for the exceptionally slow dissociation rate compared to IgG from its structurally homologous receptors20. 3-4 complex, the acute bend in the IgE-Fc, and the fact that there is very little flexibility expected between the N-terminal end of the C
3-4 complex, the acute bend in the IgE-Fc, and the fact that there is very little flexibility expected between the N-terminal end of the C 2 domains and the Fab regions, leads to a picture of the receptor-bound IgE molecule (Fig. 3c) that is remarkably similar to that drawn 16 years ago based on the FRET studies7. Therefore, it is likely that there are orientational constraints on the ligands, and location of allergenic epitopes that can effectively crosslink two or more receptor-bound IgE molecules and trigger cell activation. These effects are currently under investigation21. However, the first crystal structure of an allergen–IgE complex, that of the dimeric
2 domains and the Fab regions, leads to a picture of the receptor-bound IgE molecule (Fig. 3c) that is remarkably similar to that drawn 16 years ago based on the FRET studies7. Therefore, it is likely that there are orientational constraints on the ligands, and location of allergenic epitopes that can effectively crosslink two or more receptor-bound IgE molecules and trigger cell activation. These effects are currently under investigation21. However, the first crystal structure of an allergen–IgE complex, that of the dimeric  -lactoglobulin bound by two Fab regions of IgE, shows how the relative position of the two epitopes in this case enables crosslinking to occur22.
-lactoglobulin bound by two Fab regions of IgE, shows how the relative position of the two epitopes in this case enables crosslinking to occur22. -helical coiled-coil 'stalk' (Fig. 4a). However, the stalk region is susceptible to proteolysis, leading to the release of various soluble fragments (soluble CD23) that have activities that depend on their oligomeric state. The principal endogenous protease that releases soluble CD23 has recently been identified as ADAM10 (a disintegrin and metalloproteinase 10)23, 24. Subsequently, smaller soluble fragments of CD23 are generated of various sizes; those containing stalk sequences form trimers in a concentration-dependent manner or on IgE binding, whereas those lacking any stalk region are monomeric25. In addition to promoting oligomerization, however, the stalk region probably has other functions, as a peptide at the base of the stalk region has been implicated in binding to MHC class II molecules26. In the human form, but not the mouse form, the lectin head domains of CD23 additionally have a C-terminal 'tail' sequence (Fig. 4a). IgE-binding activity is contained within the head, but the name 'low affinity' is a misnomer; although the affinity of a single head for IgE-Fc is low (Ka
-helical coiled-coil 'stalk' (Fig. 4a). However, the stalk region is susceptible to proteolysis, leading to the release of various soluble fragments (soluble CD23) that have activities that depend on their oligomeric state. The principal endogenous protease that releases soluble CD23 has recently been identified as ADAM10 (a disintegrin and metalloproteinase 10)23, 24. Subsequently, smaller soluble fragments of CD23 are generated of various sizes; those containing stalk sequences form trimers in a concentration-dependent manner or on IgE binding, whereas those lacking any stalk region are monomeric25. In addition to promoting oligomerization, however, the stalk region probably has other functions, as a peptide at the base of the stalk region has been implicated in binding to MHC class II molecules26. In the human form, but not the mouse form, the lectin head domains of CD23 additionally have a C-terminal 'tail' sequence (Fig. 4a). IgE-binding activity is contained within the head, but the name 'low affinity' is a misnomer; although the affinity of a single head for IgE-Fc is low (Ka  106–107M-1), the avidity effect achieved with the trimer leads to an affinity (Ka
106–107M-1), the avidity effect achieved with the trimer leads to an affinity (Ka  108–109M-1) approaching the high-affinity of Fc
108–109M-1) approaching the high-affinity of Fc RI (Refs 25, 27).
RI (Refs 25, 27). |
FIGURE 4 | The structure of CD23.
a | A schematic representation of membrane-bound CD23, showing the extracellular trimeric
 -helical coiled-coil 'stalk', the three C-type lectin domain 'heads' and the C-terminal 'tails'. N-linked glycosylation sites near the base of the stalk are also shown. b | The three-dimensional structure of one of the head domains. The NMR structure (shown in blue; Protein Data Bank (PDB) ID: 1T8D) and X-ray crystal structures (PDB ID: 2H2T) are superimposed, and the latter is displayed only in the regions where the two structures differ most (shown in red); other differences occur in the N- and C-terminal regions of the domain. The single Ca2+ ion observed in each structure is indicated as a sphere in its respective colour. Binding regions are indicated for: IgE-Fc (C -helical coiled-coil 'stalk', the three C-type lectin domain 'heads' and the C-terminal 'tails'. N-linked glycosylation sites near the base of the stalk are also shown. b | The three-dimensional structure of one of the head domains. The NMR structure (shown in blue; Protein Data Bank (PDB) ID: 1T8D) and X-ray crystal structures (PDB ID: 2H2T) are superimposed, and the latter is displayed only in the regions where the two structures differ most (shown in red); other differences occur in the N- and C-terminal regions of the domain. The single Ca2+ ion observed in each structure is indicated as a sphere in its respective colour. Binding regions are indicated for: IgE-Fc (C 3 domain); CD21 (domains 1 and 2) in a region adjacent to the C-terminal tail, most of which is either absent (NMR structure) or disordered (X-ray structure); and 3 domain); CD21 (domains 1 and 2) in a region adjacent to the C-terminal tail, most of which is either absent (NMR structure) or disordered (X-ray structure); and  V V 5-integrin, in the N-terminal region close to the connection to the stalk. 5-integrin, in the N-terminal region close to the connection to the stalk. |
 3) and CD21 (N-terminal domains 1 and 2)27. The isolated C
3) and CD21 (N-terminal domains 1 and 2)27. The isolated C 3 domain was used because IgE-Fc containing both C
3 domain was used because IgE-Fc containing both C 3 domains formed complexes of very high molecular weight27. The CD21 site is incompletely represented in the NMR and crystal structures; the full C-terminal tail region was not present in the construct used in the NMR study, and although it was present in that used for the X-ray analysis, it was not visible in the structure, implying flexibility of this region. However, the IgE and CD21 binding sites are clearly distant from each other (Fig. 4b), consistent with earlier evidence from site-directed mutagenesis34, 35. Further studies showed that both could bind simultaneously27. A model for trimerization of the heads was also proposed based on the NMR data, which brings the three CD21 binding sites close together adjacent to the stalk and leaves the three IgE binding sites positioned around the periphery of the trimer of heads.
3 domains formed complexes of very high molecular weight27. The CD21 site is incompletely represented in the NMR and crystal structures; the full C-terminal tail region was not present in the construct used in the NMR study, and although it was present in that used for the X-ray analysis, it was not visible in the structure, implying flexibility of this region. However, the IgE and CD21 binding sites are clearly distant from each other (Fig. 4b), consistent with earlier evidence from site-directed mutagenesis34, 35. Further studies showed that both could bind simultaneously27. A model for trimerization of the heads was also proposed based on the NMR data, which brings the three CD21 binding sites close together adjacent to the stalk and leaves the three IgE binding sites positioned around the periphery of the trimer of heads. -barrel structure that is a constituent of many complement-related proteins. The crystal structure of domains 1 and 2 of CD21 is known36, and the whole molecule adopts an extended, flexible structure that is approximately 40 nm long37 (Fig. 5a). CD21 could clearly make extensive, multi-point attachments to the CD23 trimer, perhaps to the head as well as to the tail. However, we are still far from understanding the details of this interaction.
-barrel structure that is a constituent of many complement-related proteins. The crystal structure of domains 1 and 2 of CD21 is known36, and the whole molecule adopts an extended, flexible structure that is approximately 40 nm long37 (Fig. 5a). CD21 could clearly make extensive, multi-point attachments to the CD23 trimer, perhaps to the head as well as to the tail. However, we are still far from understanding the details of this interaction.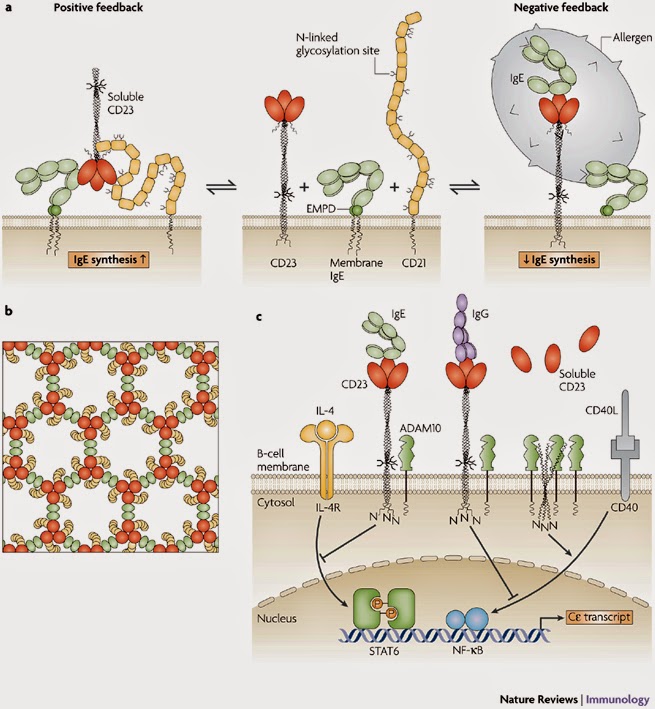 |
| FIGURE 5 | Mechanisms of IgE regulation by CD23.
a | Positive and negative regulation of IgE synthesis by human CD23. In this model, positive regulation of IgE synthesis is a result of the co-ligation of membrane IgE and CD21 on a human B cell committed to IgE synthesis by soluble CD23 released from membrane-bound CD23. IgE synthesis is thereby upregulated via synergistic signalling between the membrane IgE–Ig
 –Ig –Ig complex and the CD21–CD19 complex. (Soluble CD23 trimerizes in a concentration-dependent manner and on IgE binding, as shown here.) Negative regulation of IgE synthesis occurs by the co-ligation of IgE and CD23 on the membrane by allergen–IgE complexes. Competition between CD21 and CD23 for membrane IgE thus leads to homeostasis. b | A possible model for the formation of a signalling platform involving co-ligation of membrane IgE and CD21 by soluble CD23. It is known that: monomeric soluble CD23 binds simultaneously to a single C complex and the CD21–CD19 complex. (Soluble CD23 trimerizes in a concentration-dependent manner and on IgE binding, as shown here.) Negative regulation of IgE synthesis occurs by the co-ligation of IgE and CD23 on the membrane by allergen–IgE complexes. Competition between CD21 and CD23 for membrane IgE thus leads to homeostasis. b | A possible model for the formation of a signalling platform involving co-ligation of membrane IgE and CD21 by soluble CD23. It is known that: monomeric soluble CD23 binds simultaneously to a single C 3 domain of IgE and one molecule of CD21; IgE-Fc can bind two molecules of monomeric soluble CD23; and soluble CD23 is trimeric at the high concentrations that probably exist in tissues in allergic inflammation and on binding to IgE-Fc. Thus, trimeric soluble CD23 (shown in red) can bind three molecules of IgE (via the C 3 domain of IgE and one molecule of CD21; IgE-Fc can bind two molecules of monomeric soluble CD23; and soluble CD23 is trimeric at the high concentrations that probably exist in tissues in allergic inflammation and on binding to IgE-Fc. Thus, trimeric soluble CD23 (shown in red) can bind three molecules of IgE (via the C 3 domain; shown in green) and three molecules of CD21 (shown in yellow). Furthermore, IgE can link two molecules of soluble trimeric CD23. This may lead to the formation of an extended network, creating a signalling platform for the survival and differentiation of IgE-committed B cells. c | In this scheme, IgE binding to mouse membrane CD23 trimers (which lack the C-terminal tails that in human CD23 trimers bind CD21) interferes with the upregulation of IgE class switching induced by interleukin-4 (IL-4) and CD40 ligand (CD40L). As CD23 trimers are unstable in the B-cell membrane, their unravelling allows ADAM10 (a disintegrin and metalloproteinase 10) to cleave the stalk region, releasing soluble CD23. This cleavage of membrane CD23 allows IgE synthesis to proceed by default. The binding of IgE, or certain CD23-specific IgG antibodies that bind to the lectin head domain (such as lumiliximab), stabilize the trimer and thereby enhance negative signalling. Although the negative signalling pathway of CD23 is unknown, IL-4 and CD40L act through STAT6 (signal transducer and activator of transcription 6) and NF- 3 domain; shown in green) and three molecules of CD21 (shown in yellow). Furthermore, IgE can link two molecules of soluble trimeric CD23. This may lead to the formation of an extended network, creating a signalling platform for the survival and differentiation of IgE-committed B cells. c | In this scheme, IgE binding to mouse membrane CD23 trimers (which lack the C-terminal tails that in human CD23 trimers bind CD21) interferes with the upregulation of IgE class switching induced by interleukin-4 (IL-4) and CD40 ligand (CD40L). As CD23 trimers are unstable in the B-cell membrane, their unravelling allows ADAM10 (a disintegrin and metalloproteinase 10) to cleave the stalk region, releasing soluble CD23. This cleavage of membrane CD23 allows IgE synthesis to proceed by default. The binding of IgE, or certain CD23-specific IgG antibodies that bind to the lectin head domain (such as lumiliximab), stabilize the trimer and thereby enhance negative signalling. Although the negative signalling pathway of CD23 is unknown, IL-4 and CD40L act through STAT6 (signal transducer and activator of transcription 6) and NF- B (nuclear factor- B (nuclear factor- B), respectively, to stimulate C B), respectively, to stimulate C germline gene transcription and class switching to IgE. EMPD, extracellular membrane-proximal domain; IL-4R, IL-4 receptor. Modified with permission from Ref. 85 © (2007) Current Science Inc. germline gene transcription and class switching to IgE. EMPD, extracellular membrane-proximal domain; IL-4R, IL-4 receptor. Modified with permission from Ref. 85 © (2007) Current Science Inc. |
 3 domains distinct from the Fc
3 domains distinct from the Fc RI site38, away from the local symmetry axis of the Fc, and consistent with the observed 2:1 stoichiometry of binding25. The ability of IgE to engage two molecules of CD23, and the dual binding activity of CD23, suggests a model for the formation of extensive arrays in the cell membrane involving co-crosslinking of membrane IgE and CD21 by trimeric soluble CD23 (Ref. 27). The significance of the equilibrium shown in Fig. 5a, and the extended membrane complex (schematically indicated for one possible array in Fig. 5b) are discussed below.
RI site38, away from the local symmetry axis of the Fc, and consistent with the observed 2:1 stoichiometry of binding25. The ability of IgE to engage two molecules of CD23, and the dual binding activity of CD23, suggests a model for the formation of extensive arrays in the cell membrane involving co-crosslinking of membrane IgE and CD21 by trimeric soluble CD23 (Ref. 27). The significance of the equilibrium shown in Fig. 5a, and the extended membrane complex (schematically indicated for one possible array in Fig. 5b) are discussed below. -binding protein, but now known as galectin-3, has the ability to bind to both IgE and Fc
-binding protein, but now known as galectin-3, has the ability to bind to both IgE and Fc RI via
RI via  -galactose-containing oligosaccharide chains, thereby activating mast cells or basophils by crosslinking receptor-bound IgE, Fc
-galactose-containing oligosaccharide chains, thereby activating mast cells or basophils by crosslinking receptor-bound IgE, Fc RI or both39. Galectin-3 consists of a single carbohydrate-recognition domain (CRD) with an N-terminal region of proline-, glycine- and tyrosine-rich repeats through which it readily forms pentamers in complex with oligosaccharides40; it therefore has a unique architecture and oligomerization propensity within the family of galectins, which otherwise contain just one or two CRDs and most commonly form dimers. The crystal structure of the CRD displays the canonical fold of this family, namely a sandwich of 5- and 6-stranded
RI or both39. Galectin-3 consists of a single carbohydrate-recognition domain (CRD) with an N-terminal region of proline-, glycine- and tyrosine-rich repeats through which it readily forms pentamers in complex with oligosaccharides40; it therefore has a unique architecture and oligomerization propensity within the family of galectins, which otherwise contain just one or two CRDs and most commonly form dimers. The crystal structure of the CRD displays the canonical fold of this family, namely a sandwich of 5- and 6-stranded  -sheets, with the oligosaccharide binding site lying within a shallow surface groove41.
-sheets, with the oligosaccharide binding site lying within a shallow surface groove41. M
M 2-integrin in humans42 and mice43,
2-integrin in humans42 and mice43,  X
X 2-integrin42,
2-integrin42,  V
V 3-integrin44 and, most recently,
3-integrin44 and, most recently,  V
V 5-integrin45. The region of CD23 involved in the interaction with
5-integrin45. The region of CD23 involved in the interaction with  V
V 5-integrin has been located, and is distinct from the IgE and CD21 binding sites but close to the stalk region (Fig. 4b). It includes two adjacent basic residues in a tripeptide motif, Arg-Lys-Cys, located on an exposed disulphide-bonded loop, and is recognized by a site on the integrin
5-integrin has been located, and is distinct from the IgE and CD21 binding sites but close to the stalk region (Fig. 4b). It includes two adjacent basic residues in a tripeptide motif, Arg-Lys-Cys, located on an exposed disulphide-bonded loop, and is recognized by a site on the integrin  -chain that is distinct from the RGD sequence binding site of the integrin45. No further details of these interactions are known.
-chain that is distinct from the RGD sequence binding site of the integrin45. No further details of these interactions are known.Functions of IgE
 RI complex on mast cells, is immediate hypersensitivity. This reveals itself in characteristic signs and symptoms in the different target organs of allergy, the skin (atopic dermatitis or eczema), the nose (rhinitis), the lungs (asthma) and the gut (food allergic reactions)4, 15. Crosslinking of IgE–Fc
RI complex on mast cells, is immediate hypersensitivity. This reveals itself in characteristic signs and symptoms in the different target organs of allergy, the skin (atopic dermatitis or eczema), the nose (rhinitis), the lungs (asthma) and the gut (food allergic reactions)4, 15. Crosslinking of IgE–Fc RI complexes on the mast-cell surface by allergens leads, within minutes, to the so-called 'early phase' of the allergic reaction, which involves mast-cell degranulation and the synthesis of lipid mediators. Cytokines and chemokines liberated in this early phase initiate the 'late phase', which peaks some hours later and involves the recruitment and activation of inflammatory cells at sites sensitive to allergen. Similarly, but without overt symptoms, allergens activate the IgE-sensitized APCs, which in turn promote IgE production by B cells to replenish the IgE consumed in the allergic reaction, thereby maintaining mast-cell and APC sensitization (Fig. 6).
RI complexes on the mast-cell surface by allergens leads, within minutes, to the so-called 'early phase' of the allergic reaction, which involves mast-cell degranulation and the synthesis of lipid mediators. Cytokines and chemokines liberated in this early phase initiate the 'late phase', which peaks some hours later and involves the recruitment and activation of inflammatory cells at sites sensitive to allergen. Similarly, but without overt symptoms, allergens activate the IgE-sensitized APCs, which in turn promote IgE production by B cells to replenish the IgE consumed in the allergic reaction, thereby maintaining mast-cell and APC sensitization (Fig. 6). RI (Ref. 46). The receptor is highly expressed (
RI (Ref. 46). The receptor is highly expressed ( 500,000 copies per cell) on tissue mast cells, probably as a result of IgE-mediated upregulation of Fc
500,000 copies per cell) on tissue mast cells, probably as a result of IgE-mediated upregulation of Fc RI expression15, 47. The concentrations of IgE required for the upregulation of Fc
RI expression15, 47. The concentrations of IgE required for the upregulation of Fc RI in the mucosal tissues, which are higher than those normally present in the circulation, may derive from IgE synthesis by local B cells48. The rate of this process is more than sufficient to maintain saturation of the Fc
RI in the mucosal tissues, which are higher than those normally present in the circulation, may derive from IgE synthesis by local B cells48. The rate of this process is more than sufficient to maintain saturation of the Fc RI molecules on the mast cells4, 48, and any excess IgE is preferentially directed into secretions, rather than entering the circulation49, 50. Local IgE synthesis is important for the function of IgE, as is discussed later.
RI molecules on the mast cells4, 48, and any excess IgE is preferentially directed into secretions, rather than entering the circulation49, 50. Local IgE synthesis is important for the function of IgE, as is discussed later. RI and activate mast-cell signalling. The effective concentrations of IgE on the cell surface exceed those in solution by many orders of magnitude, and their orientation may further promote self association. The structural determinants of cytokinergic activity are under investigation. It is not yet clear whether these IgE molecules act in the same manner in vivo, and if so what the physiological consequences might be. This subject is discussed in greater detail in two recent reviews15, 51.
RI and activate mast-cell signalling. The effective concentrations of IgE on the cell surface exceed those in solution by many orders of magnitude, and their orientation may further promote self association. The structural determinants of cytokinergic activity are under investigation. It is not yet clear whether these IgE molecules act in the same manner in vivo, and if so what the physiological consequences might be. This subject is discussed in greater detail in two recent reviews15, 51. , C
, C or C
or C ), to C
), to C , in the immunoglobulin heavy-chain locus. CSR to IgE is induced by the cytokines IL-4 or IL-13 secreted by T helper 2 (TH2) cells. Until recently, CSR had been thought to occur only in the germinal centres of lymphoid tissue. The origin of the IgE-expressing B cells and plasma cells in the respiratory tract mucosa was revealed by the observation of CSR in these B cells54, 55, 56, 57, 58, 59. Moreover, somatic hypermutation (SHM) and clonal expansion of respiratory-tract B cells was observed, implying that clonal selection and affinity maturation operate in the B-cell population of the tissue54, 55, 58. The conditions of allergic inflammation may therefore shape the local antibody repertoire, favouring the production of high-affinity IgE antibodies. The synthesis of IgE directed against grass pollen allergens was found to persist beyond the grass pollen season48; this is consistent with the hypothesis that inflamed tissues, as well as the bone marrow, constitute a survival niche for antibody-secreting plasma cells60, 61.
, in the immunoglobulin heavy-chain locus. CSR to IgE is induced by the cytokines IL-4 or IL-13 secreted by T helper 2 (TH2) cells. Until recently, CSR had been thought to occur only in the germinal centres of lymphoid tissue. The origin of the IgE-expressing B cells and plasma cells in the respiratory tract mucosa was revealed by the observation of CSR in these B cells54, 55, 56, 57, 58, 59. Moreover, somatic hypermutation (SHM) and clonal expansion of respiratory-tract B cells was observed, implying that clonal selection and affinity maturation operate in the B-cell population of the tissue54, 55, 58. The conditions of allergic inflammation may therefore shape the local antibody repertoire, favouring the production of high-affinity IgE antibodies. The synthesis of IgE directed against grass pollen allergens was found to persist beyond the grass pollen season48; this is consistent with the hypothesis that inflamed tissues, as well as the bone marrow, constitute a survival niche for antibody-secreting plasma cells60, 61. RI on the surface of Langerhans cells and to the interdigitating epithelial dendritic cells, which are all engaged at our body surfaces in the surveillance for antigens in the environment. Following the capture of allergens they either migrate to local lymph nodes or possibly remain in the tissues where they present the processed allergens to TH cells67. Similarly, allergen–IgE complexes bound in the mucosa to CD23 that is expressed by allergen-activated B cells can facilitate antigen presentation to the T cells68. The process of antigen presentation by way of CD23 is termed facilitated antigen presentation (FAP). The interaction between CD23 and HLA-DR in the cell membrane is involved in the trafficking of allergen–IgE–CD23 complexes to endosomes, where the allergen-derived peptides are loaded onto the HLA-DR molecules for presentation at the B-cell surface69.
RI on the surface of Langerhans cells and to the interdigitating epithelial dendritic cells, which are all engaged at our body surfaces in the surveillance for antigens in the environment. Following the capture of allergens they either migrate to local lymph nodes or possibly remain in the tissues where they present the processed allergens to TH cells67. Similarly, allergen–IgE complexes bound in the mucosa to CD23 that is expressed by allergen-activated B cells can facilitate antigen presentation to the T cells68. The process of antigen presentation by way of CD23 is termed facilitated antigen presentation (FAP). The interaction between CD23 and HLA-DR in the cell membrane is involved in the trafficking of allergen–IgE–CD23 complexes to endosomes, where the allergen-derived peptides are loaded onto the HLA-DR molecules for presentation at the B-cell surface69. Rs and cause epitope spreading to other antigens. Indeed, CD23-mediated FAP is known to be as efficient as Fc
Rs and cause epitope spreading to other antigens. Indeed, CD23-mediated FAP is known to be as efficient as Fc R-mediated antigen presentation by dendritic cells70, orders of magnitude more efficient than B-cell internalization via surface immunoglobulin (the BCR). Furthermore, whereas FAP enhances the presentation of allergens recognized by pre-existing IgE molecules, it suppresses that of other antigens68. FAP therefore serves in a positive-feedback mechanism to enhance allergic sensitization (Fig. 7b). This is a clinically serious phenomenon, as monospecific allergies often deteriorate into polyspecific allergies.
R-mediated antigen presentation by dendritic cells70, orders of magnitude more efficient than B-cell internalization via surface immunoglobulin (the BCR). Furthermore, whereas FAP enhances the presentation of allergens recognized by pre-existing IgE molecules, it suppresses that of other antigens68. FAP therefore serves in a positive-feedback mechanism to enhance allergic sensitization (Fig. 7b). This is a clinically serious phenomenon, as monospecific allergies often deteriorate into polyspecific allergies. |
| FIGURE 7 | CD23-dependent epitope spreading.
a | Antigen X, recognized by a B-cell receptor (BCR) specific for epitope 1, is internalized, processed and presented by MHC class II molecules. T cells specific for antigen X1 participate in cognate interactions with the B cell, leading to the production of IgG antibodies specific for epitope X1, and, if followed by class-switch recombination (CSR), IgE antibodies. T cells generated in this initial process, specific for other peptide fragments (X2-specific T cells), may take part in cognate interactions with B cells specific for other epitopes (X2-specific B cells), leading to IgG and perhaps also IgE specific for epitope X2. Thus, B cells acting as antigen-presenting cells (APCs) using surface immunoglobulin 'spread' the response from one epitope to another, but only within the same antigen. b | By contrast, if the B cell that is activated by antigen expresses membrane CD23, it can internalize IgE bound to an allergen (Y) that is unrelated to antigen X and the specificity of that B cell. The figure shows a cognate interaction that leads to IgG and IgE specific for epitope X2 (as in part a), but the CD23-mediated internalization of allergen Y, to which a low titre of specific IgE antibodies must initially exist, leads to the production of allergen-Y-specific IgG and then IgE antibodies. The response thus spreads from antigen X to the unrelated allergen Y. Internalization of other allergen–IgE complexes by CD23 can further spread the response, just as 'professional' APCs bearing Fc receptors for IgG (Fc
 Rs) simultaneously process unrelated antigens and spread IgG responses from one antigen to another. CD23-mediated antigen presentation is more efficient than surface-immunoglobulin-mediated antigen presentation, and therefore can enhance a low level response to an allergen (such as allergen Y) and promote spreading to other allergens. This epitope spreading from one allergen to another may explain how certain allergic subjects deteriorate from sensitivity to only one allergen to multiple sensitivities70. Rs) simultaneously process unrelated antigens and spread IgG responses from one antigen to another. CD23-mediated antigen presentation is more efficient than surface-immunoglobulin-mediated antigen presentation, and therefore can enhance a low level response to an allergen (such as allergen Y) and promote spreading to other allergens. This epitope spreading from one allergen to another may explain how certain allergic subjects deteriorate from sensitivity to only one allergen to multiple sensitivities70. |
 RI
RI  -chain and
-chain and  -chain3.
-chain3. RI, whereas IL-4 and IL-13 stimulate the expression of CD23 by these cells. This in turn arms the cells for useful functions, such as clearance of antigen–IgE complexes, and killing and phagocytosis of pathogens (for example, helminth parasites)74 and tumour cells that bear 'foreign' antigens (for example, re-expressed neonatal antigens)75. The ability of Fc
RI, whereas IL-4 and IL-13 stimulate the expression of CD23 by these cells. This in turn arms the cells for useful functions, such as clearance of antigen–IgE complexes, and killing and phagocytosis of pathogens (for example, helminth parasites)74 and tumour cells that bear 'foreign' antigens (for example, re-expressed neonatal antigens)75. The ability of Fc RI and CD23 to mediate IgE-dependent monocyte- and eosinophil-mediated lysis of target cells and phagocytosis of the resulting cell fragments was recently shown for the first time by experiments with an IgE antibody against tumour cells75.
RI and CD23 to mediate IgE-dependent monocyte- and eosinophil-mediated lysis of target cells and phagocytosis of the resulting cell fragments was recently shown for the first time by experiments with an IgE antibody against tumour cells75. M
M 2-integrin and
2-integrin and  X
X 2-integrin on monocytes, and thereby initiate the production of tumour-necrosis factor and other pro-inflammatory cytokines (such as IL-1 and IL-6)76. Indeed, CD23 fragments and membrane-associated CD23 probably act sequentially in monocyte-mediated cell killing, the former acting first to mediate cell lysis, the latter disposing of the fragments. In carrying out their functions, monocytes and eosinophils inevitably also inflict some damage on bystander cells in the tissue. This side effect ultimately contributes to tissue remodelling and exacerbates the symptoms of chronic asthma, and to the compromised function of IgE in the defence against parasites74.
2-integrin on monocytes, and thereby initiate the production of tumour-necrosis factor and other pro-inflammatory cytokines (such as IL-1 and IL-6)76. Indeed, CD23 fragments and membrane-associated CD23 probably act sequentially in monocyte-mediated cell killing, the former acting first to mediate cell lysis, the latter disposing of the fragments. In carrying out their functions, monocytes and eosinophils inevitably also inflict some damage on bystander cells in the tissue. This side effect ultimately contributes to tissue remodelling and exacerbates the symptoms of chronic asthma, and to the compromised function of IgE in the defence against parasites74. RI molecules on these cells when they have engaged their specific targets. The pentameric molecule galectin-3 may of itself contribute through Fc
RI molecules on these cells when they have engaged their specific targets. The pentameric molecule galectin-3 may of itself contribute through Fc RI to airway smooth-muscle-cell contractile responses, and thus to airway hyper-responsiveness and remodelling in asthma.
RI to airway smooth-muscle-cell contractile responses, and thus to airway hyper-responsiveness and remodelling in asthma. RI- and CD23-expressing APCs may also capture the allergen–IgE complexes in the gut and cause the resident TH2 cells to enhance synthesis of allergen-specific IgE in the B cells (Fig. 8).
RI- and CD23-expressing APCs may also capture the allergen–IgE complexes in the gut and cause the resident TH2 cells to enhance synthesis of allergen-specific IgE in the B cells (Fig. 8). |
| FIGURE 8 | Role of CD23 on epithelial cells in the pathogenesis of food allergic disease.
a | Allergen–IgE complexes are captured by CD23 on the luminal side of the intestinal epithelium and transported into the mucosa, where they bind to Fc
 RI on mast cells and dendritic cells (DCs), causing allergic inflammation and local IgE class switching in B cells and IgE synthesis (1). This occurs in the fetus by the swallowing of amniotic fluid containing maternal allergen–IgE complexes, and may also represent the mechanism of early sensitization to food allergens in the adult intestinal tract. b | IgE synthesized by plasma cells in the mucosa are transported by CD23 on the mucosal side of the epithelium into the gut lumen, where they capture allergens and bind to CD23 on the luminal side of the epithelium (2) to be delivered to the mucosa. c | Finally, the inflammation caused by allergic reactions mediated by IgE in the mucosa damages the intestinal epithelium, disrupting the tight junctions between the epithelial cells. Free allergens can now pass between the cells and bind to the IgE-sensitized mast cells and DCs in the mucosa, exacerbating the food allergy (3). RI on mast cells and dendritic cells (DCs), causing allergic inflammation and local IgE class switching in B cells and IgE synthesis (1). This occurs in the fetus by the swallowing of amniotic fluid containing maternal allergen–IgE complexes, and may also represent the mechanism of early sensitization to food allergens in the adult intestinal tract. b | IgE synthesized by plasma cells in the mucosa are transported by CD23 on the mucosal side of the epithelium into the gut lumen, where they capture allergens and bind to CD23 on the luminal side of the epithelium (2) to be delivered to the mucosa. c | Finally, the inflammation caused by allergic reactions mediated by IgE in the mucosa damages the intestinal epithelium, disrupting the tight junctions between the epithelial cells. Free allergens can now pass between the cells and bind to the IgE-sensitized mast cells and DCs in the mucosa, exacerbating the food allergy (3). |
 |
| FIGURE 9 | Allergic sensitization and positive feedback.
The interaction of genes and environmental factors determine the risk of allergic sensitization3. By early 2006, six genes had been identified by positional cloning and over 100 by candidate gene association98, 99. This figure shows only some of the genes in which polymorphisms are directly involved in the synthesis or effector functions of IgE. Equally, there are many environmental factors associated with allergy. These include the level of exposure to allergens100; the way in which food is processed101, 102; elevated pH of the stomach contents of the fetus and young babies, or caused by antacid medications in adults103; atmospheric pollution104; respiratory syncytial virus and rhinovirus infections during infancy105, 106; Staphylococcal aureus infections, which generate superallergenic enterotoxins107, 108; and the activity of autoantibodies109, 110. Each of these factors might have only a small effect, but their interaction is likely to be crucial in the development of atopy. Once sensitization occurs, these factors continue to operate, and atopy is exacerbated by the resulting inflammation, persistent IgE synthesis and epitope spreading. FCERIB, high-affinity Fc receptor for IgE; IL, interleukin; ILR, IL receptor; STAT6, signal transducer and activator of transcription 6.
|
IgE homeostasis
 g per ml compared to 10 mg per ml for IgG, and may only be increased by about 10-fold in allergic individuals. This argues for the existence of mechanisms that regulate the anatomical distribution of IgE for optimal function and for the health of the individual (and perhaps even survival of the species), by suppressing its potential as a mediator of systemic anaphylaxis. Here, we consider recent evidence concerning the molecular mechanisms of IgE regulation through the network of its interacting proteins.
g per ml compared to 10 mg per ml for IgG, and may only be increased by about 10-fold in allergic individuals. This argues for the existence of mechanisms that regulate the anatomical distribution of IgE for optimal function and for the health of the individual (and perhaps even survival of the species), by suppressing its potential as a mediator of systemic anaphylaxis. Here, we consider recent evidence concerning the molecular mechanisms of IgE regulation through the network of its interacting proteins. RI site on the IgE is blocked by bound CD23, thereby rendering the antibody inactive. Nevertheless, IgE can exert its functions through Fc
RI site on the IgE is blocked by bound CD23, thereby rendering the antibody inactive. Nevertheless, IgE can exert its functions through Fc RI at a lower concentration range, by reason of its higher affinity for this receptor.
RI at a lower concentration range, by reason of its higher affinity for this receptor. : 0.8 kb versus S
: 0.8 kb versus S : 1.5 kb), or the DNA sequence of this region. Many double-strand breaks are introduced into the immunoglobulin heavy-chain gene locus during the process of CSR93, and the cells that fail to maintain an intact chromosome during this process undergo apoptosis. Consequently, cell division is often accompanied by cell death and few cells may survive long enough to switch to IgE.
: 1.5 kb), or the DNA sequence of this region. Many double-strand breaks are introduced into the immunoglobulin heavy-chain gene locus during the process of CSR93, and the cells that fail to maintain an intact chromosome during this process undergo apoptosis. Consequently, cell division is often accompanied by cell death and few cells may survive long enough to switch to IgE. RI (Fig. 3b, c). The bent structure of IgE may, for membrane-bound IgE, limit the possibilities for antigen binding, although the long form of IgE, with its larger EMPD (Fig. 5a) and perhaps greater conformational freedom, may have an advantage over the short membrane-tethered form in this respect12. It has been shown that crosslinking of the two isoforms leads to different kinetics of protein tyrosine phosphorylation and the ensuing cellular responses: crosslinking of the short form leads to inhibition of B-cell growth, whereas signalling by the long form is permissive for cell proliferation12.
RI (Fig. 3b, c). The bent structure of IgE may, for membrane-bound IgE, limit the possibilities for antigen binding, although the long form of IgE, with its larger EMPD (Fig. 5a) and perhaps greater conformational freedom, may have an advantage over the short membrane-tethered form in this respect12. It has been shown that crosslinking of the two isoforms leads to different kinetics of protein tyrosine phosphorylation and the ensuing cellular responses: crosslinking of the short form leads to inhibition of B-cell growth, whereas signalling by the long form is permissive for cell proliferation12.Prevention and treatment of allergic disease
 RI — is not new4, but has been slow to reach the clinic in the form of omalizumab (Fig. 6). This antibody is proving its value in an ever-widening range of allergic disorders96, but the expense of producing it is prohibitive for all but a small range of applications; it is currently approved for use only in moderate to severe asthma, and then only in cases already found refractory to other (less costly) treatments. Therefore, an attractive option will be the design of small molecule inhibitors of the IgE–Fc
RI — is not new4, but has been slow to reach the clinic in the form of omalizumab (Fig. 6). This antibody is proving its value in an ever-widening range of allergic disorders96, but the expense of producing it is prohibitive for all but a small range of applications; it is currently approved for use only in moderate to severe asthma, and then only in cases already found refractory to other (less costly) treatments. Therefore, an attractive option will be the design of small molecule inhibitors of the IgE–Fc RI interaction, based on what is known about the structural changes accompanying complex formation, to 'lock' IgE or Fc
RI interaction, based on what is known about the structural changes accompanying complex formation, to 'lock' IgE or Fc RI in an inactive conformation4, 10, 97.
RI in an inactive conformation4, 10, 97.Links
DATABASES
RCSB Protein Data Bank
1F6A | 1FP5 | 1J87 | 1O0V | 1T8D | 2H2T
Entrez-Gene
Fc RI | CD23 | galectin-3 | CD21 |
RI | CD23 | galectin-3 | CD21 |  M
M 2-integrin |
2-integrin |  X
X 2-integrin | ADAM10
2-integrin | ADAM10
Review
FURTHER INFORMATION
Allergy and asthma group homepage
Acknowledgements
About the authors
Author affiliations
- Randall Division of Cell & Molecular Biophysics, and MRC & Asthma UK Centre in Allergic Mechanisms of Asthma, King's College London, New Hunt's House, Guy's Campus, London, SE1 1UL, UK.
Email: hannah.gould@kcl.ac.uk; Email: brian.sutton@kcl.ac.uk

























